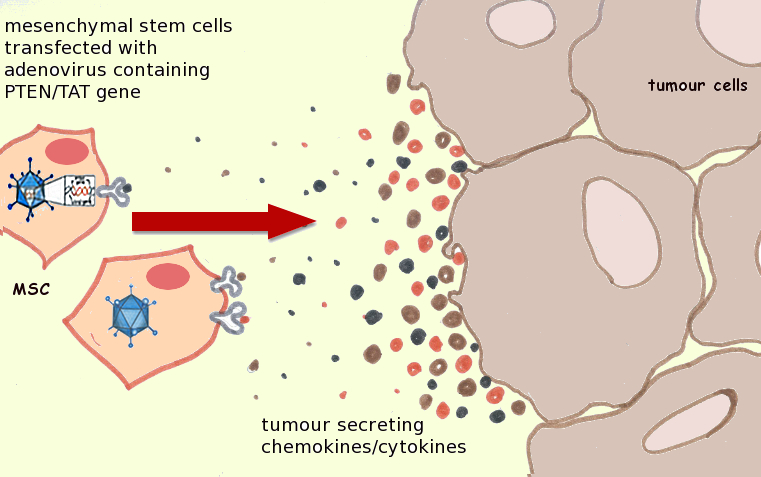
 |
| Genetically-modified MSCs home to tumor cells and accumulate at the tumor site. Image adapted from http://dx.doi.org/10.1016/j.canlet.2011.02.012. |
In the body, MSCs are known to home to sites of acute injury and
inflammation and migrate to tumors in response to tumor secretion of growth
factors, cytokines, and extracellular matrix (ECM) molecules. However, given their secretion of biomolecules
that augment new blood vessel formation, increase inflammation, and degrade the
ECM (lending to tumor metastasis), MSCs may promote rather than impede tumor
growth and migration, and confounding results from a number of in vitro and in vivo studies have been published to date. Furthermore, it has been suggested that the
ability of MSCs to interact with malignant cells and cancer stem cells might preclude their safe
therapeutic application, particularly in patients with dormant or
undiagnosed cancers. Despite these concerns, MSCs can serve as an effective
‘Trojan Horse’ for the targeted delivery of anticancer genes, proteins and
drugs to tumor cells. Such targeted
delivery can reduce the unsavory systemic side
effects that often result from the use of anti-cancer agents, reducing patient
morbidity and improving quality of life.
MSCs can serve as an effective ‘Trojan Horse’ for
the targeted delivery of anticancer genes, proteins and drugs to tumor cells.
Recently, a review
was published focusing not only on the application of MSCs for the targeted delivery
of anti-cancer agents to tumors, but also on the molecular mechanisms of MSC
accumulation in tumors, a poorly understood mechanism. For MSC-based anti-cancer therapies to be
effective clinically, these mechanisms must be understood and successfully
exploited. The authors identified
several methods to genetically-modify MSCs that resulted in tumor growth
inhibition, metastasis suppression, and prolonged survival upon MSC injection
in various tumor-laden animal models.
However, in addition to modification with anti-cancer agents, MSCs must
be able to accumulate at the site of the tumor for effective cancer eradication. The
authors postulate that increasing the accumulation efficiency of MSCs at tumor
sites can effectively target not only primary tumors but also metastatic
lesions.
Conventional thought
has dictated that cytokine secretion by inflammatory cells within the tumor
microenvironment promotes MSC recruitment and accumulation. The authors of this review, however, found
that MSC accumulation at tumor sites could not be solely accounted for by
cytokine-mediated migration.
Interestingly, they found that MSC accumulation occurred at the junction
between tumors and tumor stroma laden with blood vessels. Probing of this phenomenon revealed that MSC
accumulation in tumors occurs through MSC-tumor endothelial cell (EC) adhesion
mediated by vascular cell adhesion molecule (VCAM)-1 expression in MSCs.
They propose that the
mechanism for MSC accumulation at tumor sites is as follows:
(1) Growth factors
and chemokines recruit MSCs to the tumor microenvironment. (2) Inflammatory
cytokines, including tumor necrosis factor (TNF)-alpha, stimulate MSCs and induce
VCAM-1 expression.
(3) Activated MSCs attach
to the tumor vasculature, penetrate and accumulate at tumor sites.
Thus,
simple methods to prime MSCs prior to therapeutic administration, using commercially-available
molecules (such as TNF-alpha), may effectively promote MSC accumulation at
tumor sites and allow for efficient delivery of anti-cancer agents by
genetically-modified MSCs to tumors and metastatic lesions. Such targeted therapies could be more
successful in tumor eradication, and potentially without the systemic effects often seen
upon administration of anti-cancer drugs.
Just last year,
Mesoblast Ltd, Intrexon Corp, and ZIOPHARM Oncology, Inc announced
a partnership to develop a new class of cancer therapeutics. The press release mentions that the companies will combine Mesoblast's
proprietary Mesenchymal Lineage Cells (MLCs) with Intrexon's RheoSwitch
Therapeutic System® (RTS®) platform to deliver therapeutic transgenes to
targeted tumors at specific sites in the body to treat lung cancer. If successful, this targeted therapy with
modified MLCs could address a significant unmet clinical need. As mentioned by Mesoblast CEO, Silviu Itescu,
a key to successful development of cell-based cancer therapeutics is the
availability of clinically relevant cells
that can be used for product development efforts and be commercially
manufactured to industrial scale for off-the-shelf patient use.
These recent developments in MSC technology are opening up new lines of research in cancer, and will require high quality and well characterized MSCs to shorten the time from discovery to clinical application.
These recent developments in MSC technology are opening up new lines of research in cancer, and will require high quality and well characterized MSCs to shorten the time from discovery to clinical application.

No comments:
Post a Comment
All comments are welcome, but we do not support hateful or lewd messages. Please make your comments professional and in the spirit of adding to the scientific discussion!