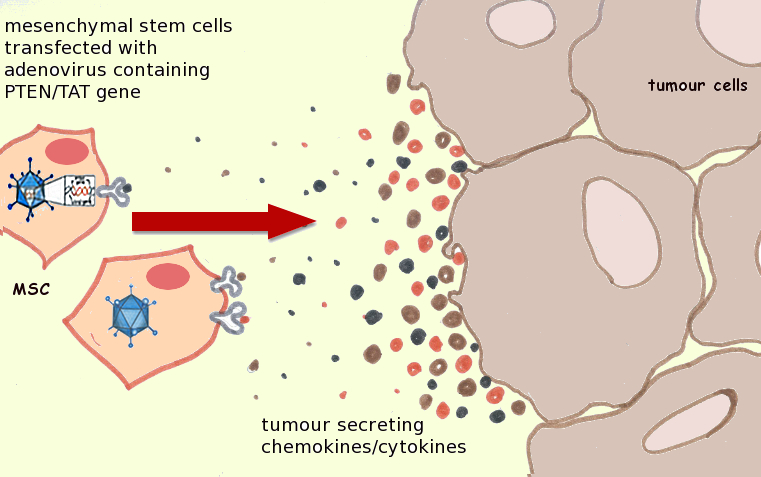
 |
| Genetically-modified MSCs home to tumor cells and accumulate at the tumor site. Image adapted from http://dx.doi.org/10.1016/j.canlet.2011.02.012. |
In the body, MSCs are known to home to sites of acute injury and
inflammation and migrate to tumors in response to tumor secretion of growth
factors, cytokines, and extracellular matrix (ECM) molecules. However, given their secretion of biomolecules
that augment new blood vessel formation, increase inflammation, and degrade the
ECM (lending to tumor metastasis), MSCs may promote rather than impede tumor
growth and migration, and confounding results from a number of in vitro and in vivo studies have been published to date. Furthermore, it has been suggested that the
ability of MSCs to interact with malignant cells and cancer stem cells might preclude their safe
therapeutic application, particularly in patients with dormant or
undiagnosed cancers. Despite these concerns, MSCs can serve as an effective
‘Trojan Horse’ for the targeted delivery of anticancer genes, proteins and
drugs to tumor cells. Such targeted
delivery can reduce the unsavory systemic side
effects that often result from the use of anti-cancer agents, reducing patient
morbidity and improving quality of life.
MSCs can serve as an effective ‘Trojan Horse’ for
the targeted delivery of anticancer genes, proteins and drugs to tumor cells.
Recently, a review
was published focusing not only on the application of MSCs for the targeted delivery
of anti-cancer agents to tumors, but also on the molecular mechanisms of MSC
accumulation in tumors, a poorly understood mechanism. For MSC-based anti-cancer therapies to be
effective clinically, these mechanisms must be understood and successfully
exploited. The authors identified
several methods to genetically-modify MSCs that resulted in tumor growth
inhibition, metastasis suppression, and prolonged survival upon MSC injection
in various tumor-laden animal models.
However, in addition to modification with anti-cancer agents, MSCs must
be able to accumulate at the site of the tumor for effective cancer eradication. The
authors postulate that increasing the accumulation efficiency of MSCs at tumor
sites can effectively target not only primary tumors but also metastatic
lesions.

