@JennWebb recently wrote an
article for the O’Reilly
Radar titled Democratizing Technology and the Road
to Empowerment. She starts out the article with a nice summary of what it means to
Democratize Technology. Jenn writes “Advancements in technology are making what once was relegated only to
highly educated scientists, engineers and developers accessible to — and
affordable for — the mainstream.“ Now, the blog she writes for is focused on
the intersection of Hardware and Software (or the “physical and digital worlds”
is how they phrase it), while we at RoosterBio are imagining a World where
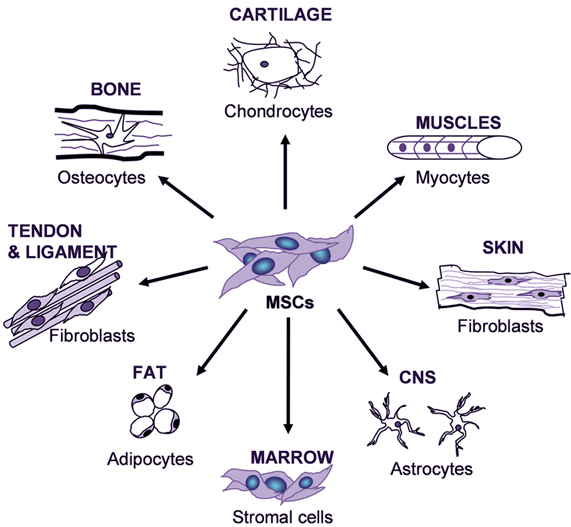
biotechnology, specifically living cellular technologies, are simplified and
cost-reduced to the point that you don’t have to be a PhD researcher in a
well-funded laboratory to perform your own experiments or build novel things
out of living cells. The concept of biology paralleling the advances of IT are well laid out elsewhere.
Today, it is much easier to incorporate living cells into your research
than it was 20 years ago. This is
evidenced by the proliferation of Cell Biology capabilities in Engineering departments
all over the world as Biomedical Engineering has turned into a formalized academic
discipline. When I was doing
undergraduate research at the University of Michigan in the early 1990’s, it took months and several
collaboration attempts before we could get living cells onto the biomaterial
constructs we were making at the time.
Today, it is more commonplace to find the tools to marry the Worlds of Cell
Biology and Engineering in the same laboratory.
Despite this, the total number of
labs with such capabilities and expertise is still very small.
We believe that the steps required to fully Democratize
Cellular Technologies will be to: