A lot of discussion on this blog
will surround MSCs. So, what is an MSC,
why are they relevant, and what’s there to talk about? A little intro:
The continuing debate on “What is an MSC”?
Adherent bone marrow-derived cells
that differentiate down various tissue lineages have traditionally been called
Mesenchymal Stem Cells, or MSCs. There
is great debate in academic circles whether this is an appropriate term, and
alternative names such as Multipotent Stromal Cells, Marrow Stromal Cells, and
even Multi-factor Secretory Cells have been proposed. While there is debate on the technical name,
there is agreement that the “MSC” acronym be maintained.
A Brief History of MSCs and their
Therapeutic Use
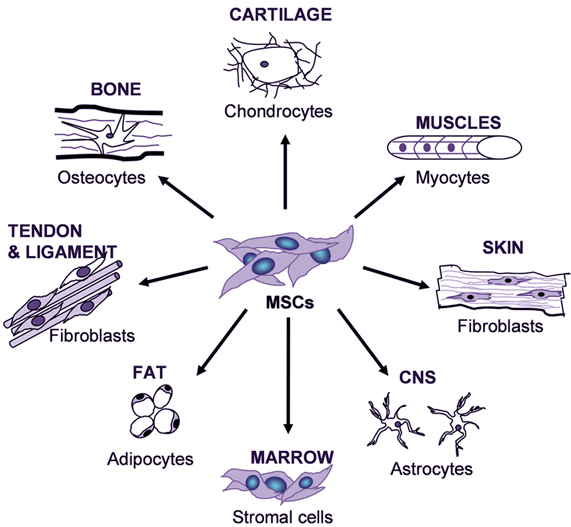 |
| MSC differentiation potential. Taken from www.sci-therapies.info. |
In the 1950s, it was discovered that
the adult bone marrow contained a rare, plastic-adherent stromal cell
population, Mesenchymal Stromal Cells (MSCs)[1,2], that participate in
maintaining the blood-forming, or hematopoietic, niche, and thus, they are
implicated in organ homeostasis, wound healing, and aging[3]. Ex vivo,
MSCs grow as long, spindle-shaped cells with prominent nuclei and are capable
of forming single-cell colonies on tissue culture plastic[4,5]. MSCs have the potential for self-renewal and
are multipotent, i.e. they have the ability to differentiate to cells from a
number of mesenchymal lineages including fat, bone, cartilage, and muscle both
in vitro and in vivo[4-7]. Thus,
these cells are also referred to as Mesenchymal Stem Cells and Mulitpotent
Stem/Stromal Cells.
Since their initial discovery in
bone marrow, MSCs have been found to reside in many tissues in the body,
including fat, umbilical cord blood, dental pulp, and peripheral blood, to name
a few. Three major criteria have been
established to define a cell as an MSC[8], including their
plastic-adherence, their surface marker profile as measured by flow cytometry,
and their ability to differentiate to osteoblasts (bone), adipocytes (fat), and
chondrocytes (cartilage) under standard in
vitro differentiation conditions. However,
these historical criteria have not kept up with how these cells are used in
application. Tri-lineage differentiation
is relevant to less than 20% of all therapeutic applications. Furthermore, flow marker expression has
recently been seen as having little relevance to function[9-11].
MSCs have been studied in a number of tissue regeneration and wound healing applications. Structural regeneration and functional improvements in damaged and diseased tissues upon therapeutic MSC application were initially attributed to cell engraftment and differentiation within target tissues. A more recent paradigm shift, however, has lead to widespread acceptance that positive outcomes following MSC (and most stem cell) transplantation are as much a function of the plethora of biomolecules secreted by these cells as a function of cell survival and differentiation[8,11]. In addition to the secretion of trophic factors, MSCs possess the ability to modulate the body’s immune response when transplanted alone or with another cell population, thereby providing for their off-the-shelf cell administration[13,14]. Given their immense therapeutic potential, relative ease of isolation, and the lack of ethical concerns surrounding use of these cells, it is no wonder that the bulk of increases in novel clinical trials since 2006 has been due to MSCs[14]. There are currently over 400 novel global clinical trials investigating MSCs as therapies across a host of diseases[15,16]. Results of clinical trials have thus far been encouraging with both allogeneic and autologous MSCs demonstrating excellent safety profiles[17]. Thus, MSCs will continue to be key components of future therapeutic agents, engineered tissues, and medical devices.
We are interested in hearing thoughts on other themes
that need to be considered related to MSCs, and if you agree or disagree with
us. Please leave a note for us in the
comments section below.
Look for an upcoming post on current limitations to MSC use, including
the need for standardization of the field.
References
1. National
Institutes of Health Stem Cell Primer; 2009.
2. Friedenstein
AJ, et al. Cell Tissue Kinet. 3:393–
403; 1970.
3. Friedenstein
AJ, et al. Transplantation. 17:331-340; 1974.
4. Caplan
AI. Prog Clin Biol Res. 217B: 307–318; 1986.
5. Pittenger
MF, et al. Science. 284:143-147; 1999.
6. Caplan
AI. J Orthop Res. 9:641– 650; 1991.
7. Wakitani
S, et al. Muscle Nerve. 18:1417–1426; 1995.
8. Dominici
M, et al. Cytotherapy. 8(4):315-317; 2006.
9. LoSurdo
JL, et al. Cytotherapy, 15(12):1527-1540; 2013.
10. Carmen J, et al. Regen Med. 7(1):85-100; 2012.
11. von Bahr
L, et al. Stem Cells. 30(7):1575-1578; 2012.
12. Gharibi B
and Hughes FJ. Stem Cells Trans Med. 1:771-782; 2012.
13. Baraniak
PR and McDevitt TC. Regen Med. 5(1):121–143; 2010.
14. Bernardo
ME and Fibbe WE. Cell Stem Cell. 13(4):392-402; 2013.
15. US Food and Drug Administration: www.clinicaltrials.gov
16. Li MD, et al. Regen Med. 9(1); 2014. doi:10.2217/RME.13.80
17. Lalu MM, et al. PLoS One. 7(10):e47559; 2012.


No comments:
Post a Comment
All comments are welcome, but we do not support hateful or lewd messages. Please make your comments professional and in the spirit of adding to the scientific discussion!